Imagen de la degeneración axonal secundaria en el sistema nervioso central.
Palabras clave:
poster, seram, degeneración, axonalResumen
Objetivos Docentes
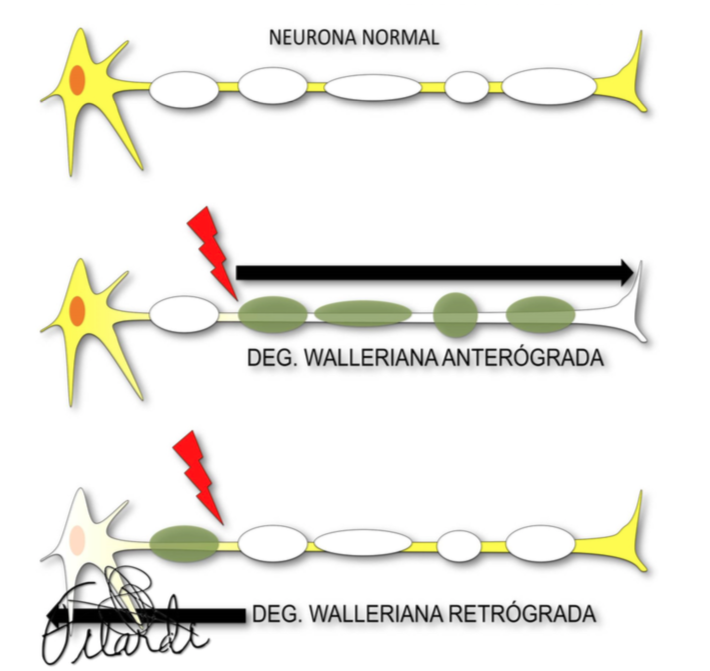
Explicar y esquematizar los tipos principales de degeneración axonal secundaria.
Repasar la anatomía y función de las principales vías que se afectan tras daños primarios cerebrales.
Ilustrar con ejemplos la degeneración axonal secundaria más frecuente que un radiólogo se encuentra en el trabajo diario.
Revisión del tema
NEUROIMAGEN DE LA DEGENERACIÓN AXONAL SECUNDARIA.
INTRODUCCIÓN.
El daño axonal secundario (DAS) o degeneración neuronal distal (DND) son términos que definen el proceso de desmielinización y desintegración de una neurona o un circuito neuronal como consecuencia de una lesión localizada a distancia.
Es una respuesta uniforme a múltiples etiologías (infarto, hemorragia, traumatismo, cirugía, neoplasias, infecciones o enfermedades desmielinizantes) que refleja un desenlace común, precedido de la desconexión funcional o diasquisis, y que consiste en cambios morfológicos que podemos valorar de manera cada vez más precoz.
Descargas
Citas
- WaIler A. Experiments on the section of the glosopharyngeal and hypoglossal nerves of the frog and observations of the alterations produced thereby in the stricture of their primitive fibres. Philos Trans R Soc Lond, Biol. 1840; 140:423-429.
- Kuhn MJ, Mikulis DJ, Ayoub DM, et al. Wallerian degeneration after cerebral infarction: evaluation with sequential MR imaging. Radiology 1989;172:179 –182.
- Inoue Y, Matsumura Y, Fukuda T, et al. MR imaging of Wallerian degeneration in the brainstem: temporal relationships. AJNR Am J Neuroradiol 1990;11:897–902.
- Tiziana De Simone, Caroline Regna-Gladin, Maria Rita Carriero, Laura Farina, and Mario Savoiardo. Wallerian Degeneration of the Pontocerebellar Fibers. AJNR Am J Neuroradiol. 2005. 26:1062–1065.
- Moon WJ, Na DG, Kim SS, et al. Diffusion abnormality of deep gray matter in external capsular hemorrhage. AJNR Am J Neuroradiol 2005;26:229 –35.
- Kuhn MJ, Johnson KA, Davis KR. Wallerian degeneration: evaluation with MR imaging. Radiology 1988;168:199 –202.
- Rabin BM, Hebel DJ, Salamon-Murayama N, Russell EJ. Distal neuronal degeneration caused by intracranial lesions. AJR Am J Roentgenol. 1998 Jul;171:95-102.
- Yamada K, Patel U, Shrier DA, Tanaka H, Chang JK, Numaguchi Y. MR imaging of CNS tractopathy: wallerian and transneuronal degeneration. AJR Am J Roentgenol. 1998 Sep;171(3):813-8.
- Innocenti GM. General organization of callosal connections in the cerebral cortex. In Jones EG, Peters A (eds) Cerebral cortex. 1986. 5 th ed. Plenum, New York, pp 291:353
- Graham DI. Hypoxia and vas- cular disorders. In: Adams JH, Duchen LW (eds) Greenfield's Neuropathology. 1992. Edward Arnold, London, pp 153:268
Weis S, Kimbacher M, Wenger E, Neu- hold A (1993) Morphometric analysis of the corpus callosum using MR: cor- relation of measurements with aging in healthy individuals. AJNR 14: 637±645
Laissy JP, Patrux B, Duchateau C, et al (1993) Midsagittal MR measurements of the corpus callosum in healthy subjects and diseased patients: a prospective study. AJNR 14: 145±154
- Meguro K, Constans JM, Courtheoux P, Theron J, Viader F, Yamadori A. Atrophy of the corpus callosum correlates with white matter lesions in patients with cerebral ischaemia. Neuroradiology. 2000 Jun;42(6):413-9.
.-Yamauchi H, Fukuyama H, Ogawa M, Ouchi Y, Kimura J. Callosal atrophy in patients with infarction and extensive leukoaraiosis: an indicator of cognitive impairment. Stroke 1994. 25: 1788±1793
Castillo M, Mukheriji SK. Early abnormalities related to postinfarction Wallerian degeneration: evaluation with MR diffusion- weighted imaging. J Comput Assist Tomogr 1999;23:1004 – 07
Kang DW, Chu K, Yoon BW, et al. Diffusion-weighted imaging in Wallerian degeneration. J Neurol Sci 2000;178:167– 69
Kinoshita T, Moritani T, Shrier DA, et al. Secondary degeneration of the substantia nigra and corticospinal tract after hemorrhagic middle cerebral artery infarction: diffusion-weighted MR findings. Magn Reson Med Sci 2002;1:175–78
MazumdarA,MukherjeeP,MillerJH,etal.Diffusion-weightedim- aging of acute corticospinal injury preceding Wallerian degenera- tion in the maturing human brain. AJNR Am J Neuroradiol 2003;24:1057– 66
Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter ar- chitecture. Neuroimage 2001;13:1174 – 85
Uchino A, Sawada A, Takase Y, et al. Transient detection of early Wallerian degeneration on diffusion-weighted MRI after an acute cerebrovascular accident. Neuroradiology 2004;46:183– 88
Nakane M, Tamura A, Miyasaka N, et al. Astrocytic swelling in the ipsilateral substantia nigra after occlusion of the middle cerebral artery in rats. AJNR Am J Neuroradiol 2001;22:660 – 63
- Kamiya K, Sato N, Nakata Y, Ito K, Kimura Y, Ota M, et al. Postoperative transient reduced diffusion in the ipsilateral striatum and thalamus. AJNR Am J Neuroradiol. 2013 Mar;34:524-32.
Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750 –57.
Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 2000;20:2369 – 82.
Moon WJ, Na DG, Kim SS, et al. Diffusion abnormality of deep gray matter in external capsular hemorrhage. AJNR Am J Neuroradiol 2005;26:229 –35.
Kitajima M, Korogi Y, Hirai T, Hamatake S, Ikushima I, Sugahara T, et al. MR changes in the calcarine area resulting from retinal degeneration. AJNR Am J Neuroradiol. 1997 Aug;18(7):1291-5.
Inouye T. Die Sehstorungen bei Schussverletzengen der kor- tikalen Sehsphare. Leipzig, Germany: Engelmann; 1909
Holmes G, Lister WT. Disturbances of vision from cerebral lesions with special reference to the cortical representation of the macula. Brain 1916;39:34-73
- Kitajima M, Korogi Y, Hirai T, Hamatake S, Ikushima I, Sugahara T, et al. MR changes in the calcarine area resulting from retinal degeneration. AJNR Am J Neuroradiol. 1997 Aug;18:1291-5.
Dai H, Mu KT, Qi JP, Wang CY, Zhu WZ, Xia LM, et al. Assessment of lateral geniculate nucleus atrophy with 3T MR imaging and correlation with clinical stage of glaucoma. AJNR Am J Neuroradiol. 2011 Aug;32:1347-53.
Cowey A, Stoerig P, Williams C. Variance in transneuronal retrograde ganglion cell degeneration in monkeys after removal of striate cortex: effects of size of the cortical lesion. Vision Res 1999;39:3642–52.
Beatty RM, Sadun AA, Smith L, et al. Direct demonstration of transsynaptic degeneration in the human visual system: a comparison of retrograde and anterograde changes. J Neurol Neurosurg Psychiatry 1982;45:143– 6.
Park HY, Park YG, Cho AH, Park CK. Transneuronal retrograde degeneration of the retinal ganglion cells in patients with cerebral infarction. Ophthalmology. 2013 Jun;120:1292-9.
Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 2009;132:628–34.
Vanburen JM. Trans-synaptic retrograde degeneration in the visual system of primates. J Neurol Neurosurg Psychiatry 1963;26:402–9.?
Cowey A, Alexander I, Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemi- anopic monkeys and humans. Brain 2011;134:2149–57.
Johnson H, Cowey A. Transneuronal retrograde degeneration of retinal ganglion cells following restricted lesions of striate cortex in the monkey. Exp Brain Res 2000;132:269–75.
Bontozoglou NP1, Chakeres DW, Martin GF, Brogan MA, McGhee RB. Cerebellorubral degeneration after resection of cerebellar dentate nucleus neoplasms: evaluation with MR imaging. Radiology. 1991 Jul;180(1):223-8.
Uchino A, Takase Y, Nomiyama K, Egashira R, Kudo S. Brainstem and cerebellar changes after cerebrovascular accidents: magnetic resonance imaging. Eur Radiol. 2006 Mar;16(3):592-7.
Decramer T, Demaerel P, van Loon J, Thijs V, MD. Wallerian Degeneration of the Superior Cerebellar Peduncle. JAMA Neurol. 2015;72(10):1206-1208.
Guillain G, Mollaret P. Deus de myoclonies synchrones et rhythmees velopharyngolaryngo-oculo-diaphragmatiques. Rev Neurol (Paris). 1931;12:545-6.
RumboldtZ, Castillo M. Hypertrophic olivary degeneration. Brain imaging with MRI and CT. En: Rumboldt Z, Castillo M, Huang B, Rossi A, editores. An image pattern approach. First edition Cambridge: Cambridge University Press; 2012. p. 132-4.
M. Blanco Ulla, A. Lo ́pez Carballeira y J.M. Pumar Cebreiro. Imagen por resonancia magne ́tica en la degeneracio ́n olivar hipertro ́fica. Radiologi ́a. 2015;57(6):505-511.
Greenfield H, Corsellis JAN, Duchen LW. General pathology of neurons and neuroglia. Neuropathology. 4.th ed New York, NY: Wiley; 1984. p. 18-9.
O’Uchi T. Wallerian degeneration of the pontocerebellar tracts after pontine hemorrhage. Int J Neuroradiol 1998;4:171–177
Fitzek C, Fitzek S, Stoeter P. Bilateral Wallerian degeneration of the medial cerebellar peduncles after ponto-mesencephalic infarc- tion. Eur J Radiol 2004;49:198–203).
Uchino A, Yuzuriha T, Murakami M, et al. Magnetic resonance imaging of sequelae of central pontine myelinolysis in chronic alcohol abusers. Neuroradiology 2003;45:877–880).
Savoiardo M, Strada L, Girotti F, et al. Olivopontocerebellar atro- phy: MR diagnosis and relationship to multisystem atrophy. Radiology 1990;174:693– 696 15.
Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998;65:65–71.
Tien RD, Ashdown BC. Crossed cerebellar diaschisis and crossed cerebellar atrophy: correlation of MR findings, clinical symptoms, and supratentorial diseases in 26 patients. AJR Am J Roentgenol. 1992 May;158(5):1155-9.
Liu Y, Karonen J, Nuutinen J, Vanninen E, Kuikka et al.. Crossed cerebellar diaschisis in acute ischemic stroke: a study with serial SPECT and MRI. Journal of Cerebral Blood Flow & Metabolism (2007) 27, 1724–1732
Lin DD, Kleinman JT, Wityk RJ. Crossed cerebellar diaschisis in acute stroke detected by dynamic susceptibility contrast MR perfusion imaging. AJNR Am J Neuroradiol. 2009;30 (4): 710-5).
Cakirer S, Basak M, Mutlu A, Galip GM. MR imaging in epilepsy that is refractory to medical therapy. Eur Radiol 2002: 12:549–558
Kim JH, Tien RD, Felsberg GJ, Osumi AK, Lee N. Clinical significance of asymmetry of the fornix and mamillary body on MR in hippo- campal sclerosis. Am J Neuroradiol 1995; 16:509–515.
Kim JH. Tien RD. FelsbergGJ, Osumi AK, Lee N. Clinical significance of asymmetry of the fornix and mamillany body on MR in hippocampal sclerosis. AJNR 1995;16:509-515.
Kodama F, Ogawa T, Sugihara S, Kamba M, Kohaya N, Kondo S et al. Transneuronal degeneration in patients with temporal lobe epilepsy: evaluation by MR imaging. Eur Radiol. 2003 Sep;13(9):2180-5.
Baldwin GN, Tsuruda JS, Maravilla KR, Hamill GS, Hayes CE. The fornix in patients with seizures caused by unilateral hippocampal sclerosis: detection of unilateral volume loss on MR images. Am JRoentgenol 1994; 162:1185–1189
Yune MJ, Lee JD, Ryu YH, Kim DI, Lee BI, Kim SJ. Ipsilateral thalamic hypoperfusion on interictal SPECT in temporal lobe epilepsy. J Nucl Med 1998. 39:281–285
Rausch R, Henry TR, Ary CM, Engel J Jr, Mazziotta J. Asymmetric interictal glucose hypometabolism and cognitive performance in epileptic patients. Arch Neurol 1994. 51:139–144.